Chapter Five
Control of corrosion is primarily an economic problem. whether of not to apply a control meathod is usually determined by the cost savings involved. The method or methods utilized must be optimum economic choice.
1- Return on investment (ROI) :-
Where:
O = annual costs including maintenance, production losses, etc.
I/n = linear depreciation .
I = Investment or installed cost.
n = anticipated life in years and subscripts a and b refer to present and proposed (or alternate) installations, respectively.
2- the net present value (NPV):
The simple economic method given above are often not a good basis of comparison. Hence, we have to deal with more rigorous analysis. To consider the schedule of payments and income, we know that value of money changes due to:
1- Interest, which reflects rent paid on the use of money.
2- Returns received from competing investments.
3- Inflation, which can be compensated in the interest rate.
The net present value (NPV) provides the most accurate basis for analyzing business costs and can be directly applied to the economics of corrosion control.
Although NPV involved extensive computations, these can easily performed with pocket calculators, especially those with programmable functions.
The concept of present value is relatively simple, it is based on the growth of an investment receiving compound interest:
We define PV as the present value of the investment and FV as its future value. Compounding the interest after a one year period gives:
Example: (1)
We have three pressure vessels as shown in the following table.
pressure vessel | Investment $ | Maintenance cost $ year | Life time (year) |
Steel | 100000 | 12000 | 2 |
Stainless | 160000 | 8000 | 4 |
Copper | 240000 | 5000 | 8 |
Select the pressure vessel based on ROI greater than 20% .
Solution:
We take steel tank as basis for comparison.
Step 1:
Calculate ROI between steel tank and stainless steel tank.
Step 2:
Calculate ROI between steel tank and copper tank.
Example: (2)
We have three pressure vessels with the informations as shown in the following table.
pressure vessel | Investment $ | Maintenance cost $ / year | Life time (year) |
Steel | 100000 | 12000 | 2 |
Stainless | 160000 | 8000 | 4 |
Copper | 240000 | 5000 | 8 |
The interest rate 10%. Select the most economical tank.
Solution:
Step1 – we take period time of all alternative equal (8 years)
Costs, dollars
Year | Steel | Stainless | Copper |
0 | 100000 | 160000 | 240000 |
1 | 12000 | 8000 | 5000 |
2 | 100000 + 12000 | 8000 | 5000 |
3 | 12000 | 8000 | 5000 |
4 | 100000 + 12000 | 160000 + 8000 | 5000 |
5 | 12000 | 8000 | 5000 |
6 | 100000 + 12000 | 8000 | 5000 |
7 | 12000 | 8000 | 5000 |
8 | 12000 | 8000 | 5000 |
Step 2:
Calculate NPV for each alternative: -
Step 3:
Select the better alternative due to VPV values, the copper tank is the most economical.
Corrosion Rate expression
A good corrosion rate expression should involve:
1- familiar units.
2- easy calculation with minimum opportunity for error.
3- ready conversion to life in years.
4- penetration.
The formula for calculating this rate is
W is the weight loss in milligrams
D is the density in g/cm3
A is the area in cm2
T is the time of exposure in hours
Conversion from other units to obtain mils per year is:
Multiply | By |
in/yr | 1000 |
In/month | 12100 |
mg/dm2/day (mdd) | 1044/ Specific gravity |
A very rough rule for checking results with respect to minimum test time (Tmin) is the formula.
This formula is based on the general rule that the lower the corrosion rate the longer the test should be run.
Proper selection of time and number of periods of exposure are important and misleading results may be obtained, if these factors are mat considered. At beast two periods should by used.
Example:
Determine the corrosion allowance for a storage tank:
The following informations of the corrosion test for an storage tank metal are abtained:
- The dimension of tested the metal strip are 50 x 50 x 4mm
- The intial weight of strip 78. 150 gm .
- The final weight is 77.452 gm .
- The exposure time for test is 360 hr.
- Life time of the tank is 15 year.
Then W = 78.150 – 77.452 = 0.698 gm .
= 698 mg
A = 2 (5x5 + 0.4x5 + 0.4x5)
= 5 8 cm2
Galvanic corrosion
Area effect:
Important factor in galvanic corrosion is the area effect, or the ratio of the cathodic to anodic areas, an unfavorable area ratio consists of a large cathode and a small anode. Corrosion of the anode may be 100 to 1000 time greater than if the two areas were the same.
Galvanic corrosion is the corrosion rate above normal that is associated with the flow of current to a less active metals (cathode) in the same environment.
The general equation for determine the corrosion rate of anode:
Where:
P= Corrosion rate of anode coupled with the cathode.
Ac= cathode area.
Aa= Anode area.
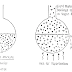
Example: Effect of area relationship on corrosion
We have two cases;
1- Copper rivets in steel plate large anode, small cathode.
| |
Cathodic Protection
This electrochemical method of corrosion control has found wide application in the protection of carbon steel underground structures such as pipe lines and tanks from external soil corrosion. It is also widely used in water systems to protect ship hulls, offshore structures, and water storage tanks.
There are two methods of producing cathodic protection for minimizing corrosion of metals.
1- The sacrificial anode method.
2- Impressed currents method.
Examples of the sacrificial anode method include the use of zinc, magnesium, or aluminum as anodes in electrical contract with the metal to protected.
The following figure illustrates cathodic protection by impressed current. Here, an external dc power supply is connected to an underground tank. The negative terminal of the power supply is connected to the tank, and the positive to an inert anode such as graphite. The electric leads to the tank and the inert electrode are carefully insulated to prevent current leakage. The anode is usually surrounded by backfill consisting of coke breeze, or bentonite which improves electric contract between the anode and the surrounding soil.
Cathodic protection of an underground tank using impressed currents.
Typical Current requirement for Cathodic protection
Comparisons of sacrificial and impressed current anodes for cathodic protection
1- Sacrificial anodes
Magnesium | Zinc | Aluminum | |
Actual consumption Ib/ampere – year | 18 | 25 | (16-20) |
Material | typical applications | Typical loss Ib/ Ampere – year |
Graphite | Soil and fresh water | 0.25-5 |
Lead | Seawater | 0.1-0.25 |
Plantinimized titanium | Seawater | Nil |
Example:
It is required to protect an underground crude oil pipeline in normal soil.
- Pipline outer diameter 14 inch
- Pipline protected length 3000 ft
- The cost of magnesium and zinc anodes are 0.9 $/Ib and 0.6 $ / Ib, respectively
- The anodes standard weight available, 25.50, and 75 Ib
solution:-
Step 1 : Determine the current density: for normal siol, the current density is 1-3 mA/ft2, we take 2 mA/ft2
Step 2: Calculate the protected area:
A= p DL
Step 4 : Determine the weight of sacrificial anodes required per year:
For Magensium = 22 x 18 =396 1b/ year
For zinc = 22 x 25 = 550 Ib/ year
We take 10 years as period of protection
For Magnesium = 396 x 10=3960 Ib
For zinc = 550 x10 = 5500 Ib
Step 5: Select the economical type of anodes:
For magnesium = 3960 x 0.9 = 3564 $
For zinc = 5500 x 0.6 = 3300 $
Select zinc due to less casts .
Step 6: Determine the anodes distribution we take the 50 Ib anode as standard weight.

























Post a Comment