Post recommended by Eng. Mohamed Fathy, the author of this post and special thanks for his efforts in enrichment our blog
Contents
This results in the vapor above the liquid being relatively rich in the lighter, more volatile material. And the liquid is left with proportionately more of the less volatile or heavier liquid. Thus a separation, to some degree, has taken place. This process is shown in Figure 2.1.
Equilibrium Stage:
Contents
2.1 Fundamentals of Separation in Towers
2.1.1 Distillation
2.1.2 Principles of Distillation
2.1.3 Reflux
2.1.4 Reboiling
2.2 Crude Distillation
2.2.1 Process Description
2.2.2 Product Specifications
2.3 Crude Distillation Operation
2.3.1 Reflux Rate Changing
2.3.2 Feed Temperature Changing
2.3.3 Side Product (Draw off) Rate Changing
2.4 Fractionator Control
2.4.1 Feed Surge Control
2.4.2 Feed Temperature (Thermal Condition)
2.4.3 Column Pressure Control
2.4.4 Reboiler Control
2.4.5 Variable Feed Tower
2.5 Operating Difficulties
2.5.1 Fouling
2.5.2 Temperature Profile
2.5.3 Operation near Critical Properties
2.5.4 Use of Grid Trays
2.5.5 Loads in Rectifying Section
2.5.6 Way of Introducing Feed
2.5.7 Reboiler
2.6 Troubleshooting Operating Problems
2.6.1 Flooding
2.6.2 Dry Trays
2.6.3 Damaged Trays
2.6.4 Water in Hydrocarbon Column
2.6.5 Foaming
2.6.6 Condenser Fogging
2.6.7 Suspect Laboratory Analysis
2.7 Glossary
Atmospheric Distillation
2.1 FUNDAMENTALS OF SEPARATION IN TOWERS
2.1.1 Distillation
Distillation is a separation process requires differences to be recognized and utilized. We separate many things by detecting a difference in a physical property, color, size, weight, shapes for example it also requires acting according to such information. Separation by distillation implies a difference in boiling points of two or more materials.
The components or compounds making up crude oil are numbered in thousands. Many of these components have similar physical properties including boiling points that may differ by only a few degrees. Therefore, it is difficult to separate some pure compounds from the complex mixture of components in crude oil by distillation alone. There are other methods of separation used in a refinery for example, extraction with a solvent, crystallization, and absorption. However distillation is the most common method. Fortunately, rarely need pure compounds and it is often enough to separate groups of compounds from each other by boiling range.
If crude oil were a final product, it would have just been a low-grade fuel struggling to establish itself against coal. If we separate the many compounds in crude oil into groups we find that these groups have characteristics that make them considerably more valuable than the whole crude oil.
Some of these groups are products some may be feedstock to other processing units where they are chemically changed into more valuable products. These products, in turn, are usually separated or purified by distillation.
2.1.2 Principles of Distillation
The basic principle of distillation is simple when a solution of tow or more components is boiled, the lighter component the one most volatile or the one with the greatest tendency to vaporize (vaporizes preferentially).
A tow component mixture, comprising crosses and dots, is contained in a vessel. We Heat is add until the more volatile material, in this case the dots, start to vaporize. Now the vapor contains a higher proportion of dots than dose the original liquid.
It is important to note that equilibrium will be established. That is, at a given temperature and pressure there is an equilibrium in composition reached. By equilibrium we mean there is a given concentration as "dots" in the vapor and in the liquid depending upon the original concentration of each component in the liquid and their respective properties in relation to each other.
 |
Figure 2.1 |
Now, let's develop this simple distillation concept into a practical operation as it is used in the refinery. First, let’s separate and remove the product (Figure 2.2).
By cooling the over head vapor, we condense and remove it from the original mixture. Thus to have made a partial separation, partial because you will note that there are a few "crosses" in the distillate product. This has occurred because at the temperature and pressure we are conducting the distillation; the heavier component still vaporizes to some extent. This is because the components of interest in a given distillation usually have fairly close boiling points.
 |
Figure 2.2 Simple Distillation |
Therefore, to purify the distillate product, we may have to conduct a second distillation as shown in Figure 2.3. Obviously, we can continue to cascade these simple distillations until we achieve the desired purity of product.
The distillations depicted so far are those we call patch, and normally practical in the refinery, although it is done frequently in the laboratory. Let us make our distillation equipment look more like refinery pieces of equipment and let us make continuous instead of patch operation.
 |
Figure 2.3 Stage Distillation |
This is called Flash Vaporization. As shown in Figure 2.4. The liquid is pumped continuously through a heater and into a drum where the pressure is lower. The lighter material flashes instantly. Vapor and liquid flow from the drum continuously. The same system is shown diagrammatically in the in the lower section of Figure. 2.4 Suppose we have 50% of the charge taken overhead. That is, we set the temperature and the pressure of the system in such a way that half the charge is boiled off. And further, suppose the resulting overhead product does not contain the desired concentration of the lighter product. As we have seen before, we can increase the purity by adding a stage of distillation.
 |
Figure 2.4 Flash Vaporization |
Suppose we add two more stages of distillation as shown in Figure 2.5. Although this is accomplishing our goal of increasing the purity of the light friction, we are also making large amounts of the intermediate product, each of which contains the same light friction.
If we compare feeds to and products from two continues stages, we note that the liquid from the upper stage and the feed to the lower stage are similar in that both are leaner in the lighter component than is the feed to the upper stage. Therefore, we could combine each indeterminate product with the feed to the next lower stage. This would improve the yield of the light fraction and all the original feed would be recovered eventually in the overhead and bottom products.
 |
| Figure 2.5 Schematic Illustration of a Typical Distillation Operation |
An obvious simplification in equipment can be made if we allow the hot vapor from the stage above the next higher (the intermediate product). This eliminates the need for the intermediate condensers and heaters. Now we have the continuous, multi-stage distillation.
Tower Sections
We have described staging for the purpose of concentrating the lighter component in the overhead. The same principles apply to concentrating the heavier component in the bottom product. The upper two stages are called rectifying stages. These below the feed are called stripping stages.
 |
| Figure 2.6 |
The upper rectifying section increases the purity of the overhead product while the stripping section increases recovery of the overhead product. In many cases, the bottom product is the one of primary interest. For the bottom, or heavy, product the rectifying section improves purity.
We now have the concept of stages. Let us define the term "stage" and see how it is actually designed mechanically.
A stage, or more specifically, an equilibrium stage, is defined as any portion of the distillation column such that the liquid and vapor leaving it have composition similar in equilibrium with each other. By definition, then, a stage should be designed in such a way as to provide intimate contact, or mixing, of the rising vapor and the descending liquid. The concept of an equilibrium stage is converted to an actual mechanical separation tray by using an efficiency factor which is less than one and depends on the tray design (Figure 2.7)
 |
Figure 2.7 Equilibrium Stage (Tray) Liquid and Vapor Loading |
The design of trays has taken many forms over the years. Some common ones are bubble cap trays, valve trays, sieve trays, uniflex trays and many others. Alternate designs include packing instead of trays. Various kinds of packing have used, some of which are pall rings, saddles and mesh. The type of column internal used depends on the application. The considerations being purity of feed, efficiency, capacity, reliability, pressure drop, liquid holdup and cost.
The column in Figure 2.6 is a simple binary column with seven trays. There is only one feed and two products, the overhead and bottoms. More complex columns may have several feed streams entering the column at different points, and more than two products. We may draw products from the side of the column. As shown in Figure 2.8 there is reflux liquid and re boiling vapor returned to the column in addition to feed.
2.1.3 Reflux
The word reflux is defined as "flowing back". Applying it to distillation tower, reflux is the liquid flowing back down the tower from each successive stage.
Kinds of Reflux (Figure 2.9)
A. Cold Reflux
Cold reflux is defined as reflux that is supplied at temperature a little below that at the top of the tower. Each pound of this reflux removes a quantity of heat equal to the sum of its latent and sensible heat required to raise its temperature from reflux drum temperature to the temperature at the top of the tower. A constant quantity of reflux is recirculated from the reflux drum into the top of the tower. It is vaporized and condensed and then returns in like quantity to the reflux drum.
B. Hot Reflux
It is the reflux that is admitted to the tower at the same temperature as that maintained at the top of the tower. It is capable of removing the latent heat because no difference in temperature is involved.
 |
Figure 2.8 Relative Amount of Reflex or Overflow Liquid at Each Tray of Contact |
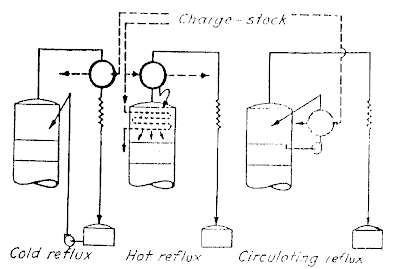 |
Figure 2.9 Methods of Removing Reflux Heat |
C. Internal Reflux
It is the reflux or the overflow from one plate to another in the tower, and may be called hot reflux because it is always substantially at its boiling point. It is also capable of removing the latent heat only because no difference in temperature is involved.
D. Circulating Reflux
It is also able to remove only the sensible heat which is represented by its change in temperature as it circulates. The reflux is withdrawn and is returned to the tower after having been cooled.
E. Side Reflux
This type of reflux (circulating reflux) may conveniently be used to remove heat at points below the top of the tower. If used in this manner, it tends to decrease the volume of vapor the tower handles.
Reflux Ratio
It is defined as the amount of internal reflux divided by the amount of top product. Since internal hot reflux can be determined only by computation. Plant operators usually obtain the reflux ratio by dividing actual reflux by the top product. It is denoted by R which equals L/D.
The Importance of Reflux Ratio
In general, increasing the reflux improves overhead purity and increases recovery of the bottom product. The number of stages required for a given separation will be dependent upon the reflux ratio used.
Two points to consider
1. A minimum number of plates (stages) required at total reflux.
2. There is a minimum reflux ratio below which it is impossible to obtain the desired enrichment however many plates are used.
Total Reflux
Total reflux is the conclusion when all the condensate is returned to the tower as reflux, no product is taken off and there is no feed.
At total reflux, the number of stages required for a given separation is the minimum at which it is theoretically possible to achieve the separation and total reflux is carried out at:
1. Towers start-up
2. The testing of the tower
Minimum Reflux
At minimum reflux, the separation can only be achieved with an infinite number of stages. This sets the minimum possible reflux ratio for the specified separation.
Optimum Reflux Ration
Practical reflux ratio will lie between the minimum for the specified separation and total reflux. The optimum value will be the one at which the specified separation is achieved at the lowest annual cost (steam or vapor). For many systems, the optimum value of reflux ratio will lie between 1:2 to 1:5 times the minimum reflux ratio.
2.1.4 Reboiling
In all our distillations discussed so far. We have added heat. Heat can be added in two ways. As we have seen, we add heat by means of the feed. We can also add heat by means of a reboiler.
The reboiler is a heat exchanger through which the bottom liquids circulate. Heat is transferred to the bottom materials which cause vaporization of the lighter components. This vapor travels up the column to provide the stripping action and the additional heat necessary to vaporize the down coming reflux.
Read More:
Atmospheric Distillation -Part 2
Atmospheric Distillation- Part 3
Atmospheric Distillation- Part 4- GLOSSARY
Atmospheric Distillation- Part 5- Appendix
Atmospheric Distillation- Part 3
Atmospheric Distillation- Part 4- GLOSSARY
Atmospheric Distillation- Part 5- Appendix










Post a Comment