Chapter Two
Corrosion in Petroleum Production Operations
THE PRODUCTION of oil and gas, its transportation and refining, and its subsequent use as fuel and raw materials for chemicals constitute a complex and demanding process. Various problems are encountered in this process, and corrosion is a major one. The costs of lost time, the replacement of materials of construction, and the constant personnel involvement in corrosion control are substantial and, if not controlled, can becatastrophic. The control of corrosion through the use of coatings, metallurgy, nonmetallic materials of construction, cathodic protection, inhibitors, and other methods has evolved into a science in its own right and has created industries devoted solely to corrosion control in specific areas.
This article will discuss the particular corrosion problems encountered, the methods of control used in petroleum production, and the storage and transportation of oil and gas to the refinery. Refinery corrosion is discussed in the article “Corrosion in Petroleum Refining and Petrochemical Operations” in this Volume.
Causes of Corrosion
The forms of corrosion commonly encountered in the petrochemical industry are discussed in the chapter “Forms of Corrosion in the Petrochemical Industry” in this Volume. This article concentrates on aspects of corrosion unique to oil and gas production.
There are several environmental factors that are more or less unique to oil and gas production. Most unique, of course, are the environments encountered in actual production formations, which, in the absence of contamination, are devoid of oxygen. In situ corrosives are limited to carbon dioxide, hydrogen sulfide, polysulfides, organic acids, and elemental sulfur. Additional unique aspects are the extremes of temperature and, particularly, pressure encountered.
In deep gas wells (6000 m, or 20,000 ft), temperatures approaching 230 0C (450 0F) have been measured, and partial pressures of CO2 and H2S of the order of 20.7 MPa (3000 psi) and 48 MPa (7000 psi), respectively, have been encountered.
Convenient access to the most important literature on H2S corrosion (particularly with regard to sulfide stress cracking) and CO2 corrosion is available in Ref 1 and 2. Supplemental information is provided in Ref 3 and 4.
To the initially oxygen-free geologic environment, a variety of oxygen-contaminated fluids may be introduced. Examples are drilling muds, which are used during drilling and maintenance of wells; dense brines; water and carbon dioxide injected for secondary oil recovery; and hydrochloric acid injected to aid formation permeability. Some of these fluids are inherently corrosive, others are potentially corrosive only when contaminated with oxygen.
Oxygen is also responsible for the external corrosion of offshore platforms and drilling rigs. In oil and gas production, highly stressed structural members are directly exposed to a corrosive seawater environment. This makes corrosion fatigue a particular concern.
Another unique aspect of oil and gas production operations, particularly in older fields, is the almost exclusive use of carbon and low-alloy steels. Only recently has any extensive use of corrosion-resistant alloys been justified.
Oxygen
Although it is not normally present at depths more than approximately 100 m (330 ft) below the surface, oxygen is nevertheless responsible for a great deal of the corrosion encountered in oil and gas production. However, oxygen-induced internal corrosion problems tend to be greater in oil production, where much of the processing and handling occurs at near-ambient pressure. This makes oxygen contamination through leaking pump seals, casing and process vents, open hatches, and open handling (as in mud pits during drilling, trucking, and so on) highly likely. Also, failure of oxygen removal processes (gas stripping and chemical scavenging) is a relatively common occurrence in water-flood systems (see the discussion “Secondary Recovery Operations” in this article).
A number of the properties of oxygen contribute to its uniqueness as a corrosive, Oxygen is a strong oxidant. This means that even trace concentrations can be harmful, and the corrosion potential of steel (almost 1.3 V) is high enough to overcome very substantial potential drops between anodic and cathodic sites. Also, the kinetics of oxygen reduction on a metal or conductive oxide surface are relatively fast. This, coupled with the low solubility of oxygen in water and brines, tends to produce conditions in which the mass transport of oxygen is the rate-limiting step in the corrosion of carbon and low-alloy steels in nonacidic environments. Mass transport is important in a number of aspects of oxygen corrosion and corrosion control. On newly installed bare steel offshore structures, mass transport of oxygen governs current requirements for cathodic protection. Poor mass transport under deposits and in crevices promotes localization of attack. In the final analysis, limiting the mass transport of oxygen plays a critical role in much of the corrosion control in oxygenated systems.
The crucial role of mass transport can be illustrated as follows. At ambient conditions, water equilibrated with air will contain on the order of 7 to 8 ppm of oxygen. Under such conditions, mass-transport-limited rates of general corrosion of steel range from about 0.25 mm/yr (10 mils/yr) in a stagnant system to 15 mm/yr (600 mils/yr) in a highly turbulent one. However, by chemically scavenging the oxygen concentration down to the order of 7 to 8 ppb, the corresponding rates are reduced to less than about 0.01 mm/yr (0.4 mils/yr).
Such rates are acceptable. However, under these conditions, magnetite forms as a stable protective corrosion product film and further lowers the corrosion rate by introducing a slower, anodically controlling step.
An even more fundamental role of magnetite should be acknowledged. This is the protection of the steel surface from reaction with water or the hydrogen ions contained in the water. Therefore, if an excess of a chelating agent such as ethylenediamine tetraacetic acid (EDTA) dissolves a protective magnetite film (Fe3O4), as would normally occur in regions of high turbulence, rapid corrosion ensues, despite the absence of oxygen.
Hydrogen Sulfide, Polysulfides, and Sulfur
Hydrogen sulfide, when dissolved in water, is a weak acid and is therefore corrosive because it is a source of hydrogen ions. In the absence of buffering ions, water equilibrated with 1 atm of H2S has a pH of about 4. However, under high pressure formation conditions, pH values as low as 3 have been calculated.
Hydrogen sulfide can also play other roles in corrosion in oil and gas production. It acts as a catalyst to promote absorption by steel of atomic hydrogen formed by the cathodic reduction of hydrogen ions. This accounts for its role in promoting sulfide stress cracking of high-strength steels (yield strength greater than approximately 690 MPa, or 100 ksi) (Ref 5).
Hydrogen sulfide also reacts with elemental sulfur. In a gas phase with a high H2S partial pressure, sulfanes (free acid forms of a polysulfide) are formed so that elemental sulfur is rendered mobile and is produced along with the remaining gaseous constituents. However, as the pressure decreases traveling up the production tubing, the sulfanes dissociate and elemental sulfur precipitates. Various solvent treatments are used to avoid plugging by such sulfur.
In the aqueous phase, under acidic conditions, sulfanes are also largely dissociated in H2S and elemental sulfur. However, enough strongly oxidizing species can remain either as polysulfide ions or as traces of sulfanes to play a significant role in corrosion reactions. Oxygen contamination of sour (H2S-containing) systems can also result in polysulfide formation.
Iron sulfide corrosion products can be important in corrosion control. Because of the low solubility, rapid precipitation, and mechanical properties of such corrosion products, velocity effects are generally not encountered in sour systems. Satisfactory inhibition at velocities up to 30 m/s (100 ft/s) has been proved.
The great range of possible iron sulfide corrosion products and their possible effects on corrosion have been extensively studied (Ref 6-9), but little of immediate practical value has resulted. At lower temperatures and H2S partial pressures, an adequately protective film often forms. The absence of chloride salts strongly promotes this condition, and the absence of oxygen is absolutely essential.
At the high temperatures (150 to 230 0C, or 300 to 450 0F) and H2S partial pressures (thousands of pounds per square inch) encountered in deep sour gas wells, a so-called barnacle type of localized corrosion (Ref 10) can occur, resulting in corrosion rates of several hundred mils per year (Ref 1 1). This type of attack is strongly promoted by polysulfide-type species and requires the presence of some minimum chloride concentration.
Although initially recognized in deep sour well environments, this same mechanism may operate at lower rates under much milder conditions.
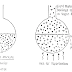
In the barnacle mechanism (Fig. 1), corrosion can be sustained beneath thick but porous iron sulfide deposits (primarily pyrrhotite, FeS) because the FeS surface is an effective cathode. The anodic reaction beneath the FeS deposit is dependent on the presence of a thin layer of concentrated FeCl2 at the Fe/Fe S interface. This intervening FeCl2 layer is acidic due to ferrous ion hydrolysis, thus preventing precipitation of FeS directly on the corroding steel surface and enabling the anodic reaction to be sustained by the cathodic reaction on the external FeS surface.
Carbon Dioxide
Carbon dioxide, like H2S, is a weakly acidic gas and becomes corrosive when dissolved in water. However, CO2 must first hydrate to carbonic acid (H2CO3) (a relatively slow reaction) before it is acidic. There are other marked differences between the two systems. Velocity effects are very important in the CO2 system; corrosion rates can reach very high levels (thousands of mils per year), and the presence of salts is often unimportant.
Whether or not corrosion in a CO2 system is inherently controlled or uncontrolled depends critically on the factors governing the deposition and retention of a protective iron carbonate (siderite, FeCO3) scale. On the other hand, there are the factors that determine the rate of corrosion on bare steel. These latter factors govern the importance of maintaining corrosion control.
Bare steel corrosion rates can be calculated from Eq 1, which was developed on the basis of electrochemical studies of the aqueous CO2/carbon steel system (Ref 12):
+ 0.67 log P- (Eq.1)
where R is the corrosion rate, t is temperature (oC), A is a constant, and P- is C02 partial pressure. When R is calculated in millimeters per year and ~ is in
atmospheres, A = 7.96. When R is calculated in mils per year and p- is in pounds per square inch, A = 8.78. Corrosion rates calculated with Eq 1 reach 25 mm/yr (1000 mils/yr) at 65oC (150 oF) and 1 MPa (150 psi) CO2 pressure, and 250 mm/yr (10 000 mils/yr) at 82 oC (180 oF) and 16 MPa (2300 psi) CO2 pressure. Obviously, such rates are on acceptable. An alternative, idealized condition occurs when a protective carbonate scale is present and when the corrosion rate is limited by the need to replenish the film lost due to solubility in the aqueous phase. Under such conditions, the rates calculated for a hypothetical sweet (CO2-containing) gas well reached a maximum of about 0.15 mm/yr (6 mils/yr) as compared to calculated bare metal rates of 500 to 2000 mm/yr (20 000 to 80000 mils/yr) (Ref 13).
Conditions favoring the formation of a protective iron carbonate scale are:
• Elevated-temperature (decreased scale solubility, decreased CO2 solubility, and accelerated precipitation kinetics)
• Increased pH, as occurs in bicarbonate-containing waters (decreased solubility)
• Lack of turbulence
Turbulence is often the critical factor in pushing a sweet system into a corrosive regime. Excessive degrees of turbulence prevent either the formation or retention of a protective iron carbonate film.
The critical velocity equation has been used to estimate when excessive turbulence can be expected in a CO2 system (Ref 14). There is no doubt that the velocity effect is real, but there is some question as to whether this exact form of the equation is the appropriate one:
where velocity is calculated in feet per second, K is a constant, and p is the density of the produced fluid (liquid + gas combined). When p is in kilograms per cubic meter, K = 7.6; for p in pounds per cubic foot, K = 100.
When both H2S and CO2 are present, simplified calculations indicate that iron sulfide may be the corrosion product scale when the H25/CO2 ratio exceeds about 1/500 (Ref 13); sour system considerations would then be expected to apply. Even in a strictly CO2 system, iron carbonate may not always be the corrosion product. Magnetite may form instead. Figure 2 shows the stability fields expected for siderite and magnetite as a function of the redox potential (expressed here in terms of hydrogen fugacity) of the system (Ref 13). In actual experience, corrosion product scales are often found to consist of mixtures or layers of siderite and magnetite.
Iron carbonate lacks conductivity and therefore does not provide an efficient cathode surface. Thus, the types of pitting mechanisms found in oxygenated and in H2S-containing systems do not occur. ,Rather, generalized corrosion occurs at any regions not covered by the protective scale. The result is that on any bare metal the anodic and cathodic regions are so microscopically dispersed that salt-to provide conductivity-is not needed to achieve the corrosion rates predicted by Eq 1.
Strong Acids
Strong acids are often pumped into wells to stimulate production by increasing formation permeability. For limestone formations, 15 and 28% hydrochloric acids are commonly used. For sandstones, additions of 3% HF are necessary.
In deep sour gas wells where HCl inhibitors lose effectiveness, 12% formic acid has been used.
Corrosion control is normally achieved by a combination of inhibition and limiting exposure time between 2 and 12 h. If corrosion-resistant alloys are present (austenitic and duplex stainless steels, and so on), concern for stress-corrosion cracking (SCC) and inhibitor ineffectiveness (respectively) may rule out the use of HC1.
Concentrated Brines
Dense halide brines of the cations of calcium, zinc, and, more rarely, magnesium are sometimes used to balance formation pressures during various production operations. All can be corrosive because of dissolved oxygen or entrained air. In addition, such brines may be corrosive because of the acidity generated by the hydrolysis of the metallic ions, as illustrated in Eq 3:Corrosivity due to acidity is worst with dense zinc brines. More expensive calcium bromide brines are now often used at densities above about 1.7 g/cm3 (14 lb/gal) (attainable with CaBr2 brines) to avoid long-term exposure to ZnCI2 brines.
Stray-Current Corrosion
If an extraneous direct current (dc) in the earth is traversed by a conductor, part of the current will transfer to the lower-resistance path thus provided. Direct currents are much more destructive than alternating currents (ac); an equivalent ac current causes only about 1% of the damage of a dc current (Ref 15). Regions of current arrival (where electrons depart) will become cathodic, and those regions where the current departs will become anodic. With corrodible metals such as carbon and low-alloy steels, corrosion in the anodic areas is the result. For example, 1 A yr can corrode 9kg (20 lb) of steel.
Cathodic protection systems are the most likely present-day sources of stray dc currents in production operations. More detailed discussions are available in Ref 4 and 16. The section “General Corrosion” of the article “Forms of Corrosion in the Petrochemical Industry” in this Volume also contains information on the causes and mechanisms of stray-current corrosion.
Under-Deposit (Crevice) Corrosion
This is a form of localized corrosion found almost exclusively (if not exclusively) in oxygen-containing systems. Such corrosion is usually most intense in chloride-containing systems. it is essential to have some form of shielding of an area on a metal such that it is wet by an electrolyte solution but is not readily accessible to oxygen, the diffusing Corrosive species.
This type of attack is usually associated with small volumes of stagnant solution caused by surface deposits (sand, sludge, corrosion products, bacterial growth), crevices in joints, and gasket surfaces. Crevice corrosion is discussed in Ref 17, and a quantitative treatment of crevice corrosion (particularly of stainless steels) is provided in Ref 18 to 20.
The mechanism of crevice corrosion hinges upon the environmental conditions resulting from the loss of hydroxide production with cessation of the cathodic reaction when the initial oxygen in the shielded region is exhausted. Thus, in the shielded region, the anodic corrosion reaction continues because the corrosion potential is maintained by the reduction of oxygen on the outside surface. However, chloride or other anions now migrate into the developing anodic region to maintain electroneutrality. Thus, a relatively concentrated, essentially ferrous chloride solution accumulates in the shielded region. As a result of the hydrolysis of the ferrous ions; the pH drops to a value of 2 or 3.
At this point, the crevice corrosion type of localized attack is fully established. The anodic reaction continues in the shielded region because in the low-pH environment the ferrous ions go readily into solution and have little tendency to precipitate as an oxide or hydroxide on the surface and thus stifle the anodic reaction. Outside, the cathodic reaction continues unperturbed. Because of the large ratio of cathodic-to-anodic surface area, high rates of localized corrosion can be maintained with very modest cathodic current densities. The mechanisms of crevice corrosion are explained in greater detail in the section “Localized Corrosion” of the article “Forms of Corrosion in the Petrochemical Industry” in this Volume.
Galvanic Corrosion
When two dissimilar metals are electrically coupled both electronically by a metal bond and ionically through an electrolyte-the more active (electronegative) metal tends to become a sacrificial anode and supply cathodic protection to the more noble metal. Such situations are often encountered in heat exchangers in which carbon steel tube sheets are used with copper alloy tubes and at junctions between piping, fasteners, or corrosion-resistant sheeting with containers of another material.
Problems with galvanic corrosion are the most acute when the cathode-to-anode area ratio is large. Such situations are often encountered inadvertently. This has happened when the normal polarity difference between zinc and steel in a galvanized pipe reversed in a bicarbonate/chloride brine so that the steel pipe walls at pinholes in the galvanizing perforated rapidly while trying to protect the extensive adjacent galvanized area. Another situation is when plastic-coated steel is coupled to more noble metal. At any pinholes in the coating, a very adverse area ratio will exist, and rapid corrosion rates can result. The section “General Corrosion” of the article “Forms of Corrosion in the Petrochemical Industry” in this Volume contains more information on this form of attack.Biological Effects
The most important biological effect on corrosion in oil and gas production is the generation of H2S by sulfate-reducing bacteria (SRB) (Desulfovibrio Desufuricans). These are anaerobic bacteria that metabolize sulfate ions (using an organic carbon source) and produce hydrogen sulfide. They can thus introduce H2S and all its corrosive ramifications into normally H2S-free systems.
Colonies of sulfate-reducing bacteria can also form deposits that are conducive to under-deposit corrosion. Contrary to previous beliefs, any resultant corrosion appears to be due to a mechanical shielding action, rather than any depolarizing action resulting from the metabolic processes of the SRB. However, this is not to deny that the introduction of H2S (whatever the source) into a crevice region could have an accelerating effect on corrosion, because H2S is known to be an anodic stimulant. More information on biological corrosion is available in the section “Localized Corrosion” of the article “Forms of Corrosion in the Petrochemical Industry” in this Volume.
Mechanical and Mechanical Corrosive Effects
Cavitations. This metal removal-often grain by grain-is due to high-pressure shock wave impingement resulting from the rapid collapse of minute bubbles created under certain conditions in high-velocity fluid handling equipment. It is usually found on pump impellers operating with too low a suction pressure.
Erosion. Most commonly, this is direct metal removal by the cutting action of high-velocity abrasive particles. Erosion failures (washouts) are seen in drill pipe when leaks (loose connections or a corrosion fatigue crack) allow drilling mud to flow through the wall under a high-pressure gradient. Erosion of flow lines at bends and joints by produced sand is probably the other most common occurrence of erosion in oil and gas production.
Erosion-Corrosion. Strictly speaking, in erosion-corrosion, only the protective corrosion product film is removed by erosive forces; however, with the protective film absent, corrosion can occur at a greatly accelerated rate. Erosion-corrosion may play a role in CO2 corrosion (Ref 21), and sand, under mild flow conditions, may also cause erosion-corrosion. Erosion-corrosion has also been noted in heavy anchor chains where their use in an abrasive bottom mud allowed corrosion at contact regions to proceed at a rate of many hundreds of mils per year.
Corrosion fatigue results from subjecting a metal to alternating stresses in a corrosive environment. At points of greatest stress, the corrosion product film becomes damaged during cycling, thus allowing localized corrosion to take place. Eventually, this leads to crack initiation and crack growth by a combination of mechanical and corrosive action. Because of this combined action, damage per cycle is greater at low cycling rates, where corrosion can play a larger role. Also, in corrosion fatigue, a fatigue limit does not exist; rather than leveling out as in simple fatigue, the usable stress level continues to decrease with increasing cycles.
The greatest concern for corrosion fatigue arises in connection with highly stressed, submerged, offshore structures. Welded connections on drill ships and on drilling and production platforms are particularly susceptible to this form of structural impairment. More information on at-tack resulting from combined corrosion and mechanical effects is available in the section “Mechanically Assisted Degradation” of the article “Forms of Corrosion in the Petrochemical Industry” in this Volume.
Environmental Stress Cracking
In corrosion-resistant alloy selection, environmental stress cracking normally requires the greatest portion of experimental effort. This follows from the use of high-strength alloys in environments in which there is relatively little experience with these alloys.
The problem is complicated by the fact that the lowest alloy content and the maximum reliable strength level are needed to achieve an economically viable choice. Because this involves design close to the limits of the material, it is necessary to define these limits as accurately as possible.
The presence of hydrogen sulfide and car-boa dioxide in hydrocarbon reservoirs can create significant materials problems. Materials degradation can take many forms, including sulfide-stress cracking, hydrogen-induced cracking, and stress-corrosion cracking. Each must be understood if appropriate measures are to be taken to minimize its effect in field operations.
Sulfide-Stress Cracking (SSC)
Sulfide-stress cracking can occur when H2S is present in the reservoir and is in contact with high-strength steels commonly used in drilling, completing, and producing wells. Sulfide-stress cracking is an embitterment phenomenon in which failures can occur at stresses well below the yield strength of the material. For SSC to occur, three conditions must be met. The first is that a surface tensile stress must be present. It is important to remember that tensile stresses can be both applied and residual. The second requirement is that the particular material must be susceptible. In oil- and gas-production environments, this includes some of the standard casing and tubing alloys, such as API 5AX, grade P-l 10. The third requirement is that an embrittling agent-in the case of SSC, hydrogen sulfide-must be present in the environment.
Sulfide-stress cracking is basically a hydrogen-embitterment phenomenon. Atomic hydrogen enters the steel to cause cracking. Many mechanisms have been proposed to explain this occurrence (Ref 22, 23). The hydrogen is generated on the surface of the steel because of a corrosion reaction. Iron reacts with H2S to form iron sulfide and hydrogen (Ref 23). This hydrogen is generated in atomic form on the steel (or sulfide) surface, where it can either combine to form molecular hydrogen and leave the surface as bubbles or diffuse into the steel. This latter process may result in hydrogen embitterment or SSC. Hydrogen sulfide prevents hydrogen recombination and thus promotes entry of atomic hydrogen into the steel. It is important to note that water must be present for this mechanism to occur; without it, SSC will not be observed, because the ionization of the hydrogen sulfide is required. Figure 3 shows an example of SSC in API grade L-80 downhole production tubing.
A number of factors influence the SSC resistance of steels. Some of these include H2S concentration, pH, temperature, strength level, and cold work.
Effect of Hydrogen Sulfide Concentration.
In general, lower H2S concentrations take longer to promote cracking than higher concentrations; lower concentrations also require high-strength materials before SSC is observed. Figure 4 illustrates typical SSC data as a function of H2S concentration for alloy steels used in oil field tubular components. The data shown in Fig. 4 and 5 are for high-strength steels covered under the following American Petroleum Institute specifications:
• 5A: “Welded or Seamless Steel Pipe for Oil or Gas Well Casing, Tubing, or Drill Pipe”
• SAC: “Welded or Seamless Steel Pipe with Restricted Yield-Strength Range
• 5AX: “High-Strength Seamless Steel Pipe for Oil or Gas Well Casing, Tubing, or Drill Pipe”
Certain proprietary grades are also included.
The concentration of hydrogen sulfide in a produced fluid, for example, brine, is a function of the hydrogen sulfide partial pressure in the gas phase, which is a function of the total gas pressure. It should be noted that in reservoirs containing small amounts of H2S there may become difficulty in obtaining an accurate analysis; the reaction of H2S with tubular goods and Other components could result in a reading that is too low. Readings
Effect of pH. The tendency toward SSC is a function of the pH of the system. With decreasing pH, the corrosion rate of the steel tends to increase, which causes more hydrogen to be produced. This causes more hydrogen to enter the steel and increases the susceptibility to cracking. It is generally agreed that increasing the pH above 8 is beneficial in reducing the tendency toward SSC. Although fluid pH control cannot be easily accomplished with produced fluids, control of drilling environments is common. During drilling operations in sour reservoirs, the pH is usually maintained in the 10 to 11 range, thus providing the opportunity to use high-strength steels.
Effect of temperature has been found to have a substantial effect on SSC resistance. As temperature increases, the resistance to SSC also increases. This is due to a reduction in the hydrogen-permeation rate at elevated temperatures (Ref 25). This effect allows materials that are susceptible to SSC at room temperature to be used at elevated temperatures. It has been found that SSC is most severe at room temperature; below room temperature, resistance to SSC again -begins to increase. The influence of temperature on SSC is illustrated in Fig. 5.
The beneficial effect of temperature on SSC resistance is of practical significance in completing wells. For example, API, grade P-I 10, steel is susceptible to SSC at room temperature and therefore is not used in such applications. However, when the temperature is kept above about 80 0C (175 oF), P-l 10 can be successfully used. Therefore, this grade of steel is often used in the deeper portions of a well, where the temperatures are higher. However, caution must be exercised because SSC would be expected to occur if the temperature should ever decrease.
Effect of Strength Level. Various metallurgical variables can be controlled to maximize the resistance of a material to SSC. The strength level (commonly measured nondestructively by hardness) is probably the most widely used criterion in ensuring that steels and stainless steels do not fail by SSC.
In most cases, carbon and low alloy steels are used at hardness’s of 22 HRC or below. The exception is the quenched-and-tempered AISI 4lxx series, which has been used at hardness’s to 26 HRC. Materials should be pretested in the anticipated service environment if there is any doubt about their suitability.
SCC resistance is influenced by steel micro-structure, which in turn depends on steel composition and heat treatment. It has been observed that a martensitic structure provides better SSC resistance than other microstructures. Figure 6 illustrates this for a molybdenum-niobium modified AISI 4135 steel (compositions of the steels discussed in Fig. 6 to 8 are given in Table 1). The data in Fig. 6(a) were developed by using simple beam specimens strained in three-point bending for measuring a critical stress, Sc, and the data in Fig. 6(b) were obtained from double-cantilever beam specimens strained by wedge opening loading for measuring a threshold stress intensity, Thus, it is important to select an alloy steel that has sufficient harden ability to achieve 100% martensite for a given application.
Furthermore, proper tempering of marten-site is essential in order to maximize SSC resistance. Figure 7 illustrates the effects of tempering temperature on SSC behavior. It is evident that higher tempering temperatures improve SSC performance. The presence of untempered martensite, however, is extremely detrimental to SSC resistance. This is illustrated in Fig. 8, which shows the effect of tempering above the Ac1 temperature for molybdenum-niobium modified 4130 steels containing two levels of silicon. Water quenching from above the Ac1 temperature results in untempered martensite with a subsequent loss in SSC resistance. It has also been found that the development of a fine prior-austenite grain size and the use of accelerated cooling rates after tempering improve SSC resistance. The necessity for adequate harden ability is quite evident when considering alloy steels for heavy section wellhead components. Figure 9 shows how the SSC resistance of conventional steels used in wellhead equipment can be improved through modifications in composition, which increase harden ability.
It is common practice when quenching tubular products for this application to implement both inside-diameter and outside-diameter quenches to ensure that the entire cross section is transformed to martensite.
Effect of Cold Work. It is widely known that cold work can adversely affect the SSC resistance of materials. The hardness is locally increased, and residual stresses can also be generated. Hardness’s that greatly exceed 22 HRC (some as high as 40 HRC have been measured) can be produced by improper straightening and handling. Even identification stamping has been reported to cause enough cold work to initiate sulfide-stress cracks.
Effect of a Stress. Because SSC is a stress-dependent phenomenon, the actual stress to which the component is subjected will affect the SSC resistance of the material. It must be remembered that the total stress, which includes both applied and residual stresses, must be considered. It is generally agreed that there is a threshold stress below which SSC is not expected. The threshold stress is a function of the material as well as environmental parameters. However, it is difficult to design to this threshold stress because of the uncertainty of controlling the service conditions at all times. Another factor that contributes to high stresses and the initiation of sulfide-stress cracks is the presence of stress concentrations, such as those found in threaded connections.
Hydrogen-Induced Cracking (HIC)
Hydrogen-induced cracking, also called step-wise cracking or blister cracking, is primarily found in lower-strength steels, typically with tensile strengths less than about 550 MPa (80 ksi). It is primarily found in line-pipe steels.
This type of degradation also begins with a reaction between steel and hydrogen sulfide in the presence of water. Again, hydrogen atoms enter the steel, but with HIC, as opposed to SSC, these hydrogen atoms combine to form hydrogen gas at internal defects. These internal discontinuities can be hard spots of low-temperature transformation products or laminations. However, manganese sulfide inclusions are the primary sites for this to occur. These inclusions tend to become elongated during pipe manufacture and give rise to high stresses at the tip of the inclusion when hydrogen gas forms there. As cracks initiate and propagate, they begin to link up with others, and a series of stepwise cracks can propagate through the material (Fig. 10). An applied stress is not required for this mechanism to occur.
Aside from reducing the amount of hydrogen being generated by reducing the corrosion reaction, another way of controlling HIC is through material processing. Shape control of sulfide inclusions is perhaps the best way to minimize the tendency toward HIC in line-pipe steels. Elongated manganese sulfide inclusions promote crack initiation and propagation due to the high stresses at the tips of the inclusions. However, the addition of calcium or rare earths to the steel makes the sulfides spherical, and because of their hardness, they remain spherical after processing. In addition, reduction of the sulfur content is also beneficial in reducing the susceptibility of steels to HIC. Other alloying additions that reduce hydrogen permeation, such as copper up to about 0.25%, are also beneficial (Ref 28).
Stress-Corrosion Cracking (SCC)
With this mechanism of degradation, a tensile stress is again required, together with a susceptible material and an environment that promotes cracking. In the oil and gas industries, the materials that are most generally found to be susceptible to SCC are the austenitic stainless steels and nickel-base alloys. Many different mechanisms have been proposed for SCC. The differences among these mechanisms depend upon the material and the environment, which would suggest that a single unified theory probably does not exist.
Austenitic stainless steels and nickel-base alloys are used in oil and gas production because they form protective films and therefore have very low corrosion rates. However, chloride ions (hence the term chloride stress-corrosion cracking), either in combination with hydrogen sulfide or alone, can attack this film, causing small pits to form. These small pits act as anodes, while the remainder of the oxide film acts as a cathode; the unfavorable area ratio causes the pit to grow. Also, the solution inside the pit is acidified because of the corrosion reaction, which also tends to increase the corrosion rate. Finally, a crack is initiated at the base of the pits because of the stress concentration, and propagation occurs because of the tensile stress. The crack often grows along grain boundaries because grain boundaries are electrochemically more active than the bulk grains. This is called active-path corrosion and is one of many possible mechanisms. Chloride 5CC is usually observed at temperatures exceeding 65 to 95 “C (150 to 200 “F). Figure 11 shows an example of chloride Sec in an austenitic stainless steel. More information on 5CC is available in the section “Environmentally Induced Cracking” of the article “Forms of Corrosion in the Petrochemical Industry” in this Volume.
There has been considerable interest in minimizing weight-loss corrosion in sour environments by using corrosion-resistant alloys. Corrosion-resistant alloys include nickel-base alloys, austenitic stainless steels, and duplex stainless steels. Use of these alloys is extremely attractive for offshore applications, where the corrosion inhibition of carbon and low-alloy steels may be difficult and expensive. Corrosion-resistant alloys possess very low corrosion rates because of the presence of a passivating oxide layer that protects the base metal from further corrosion. In general, it has been found that the risk of 5CC of these alloys in production environments increases as temperature and chloride and hydrogen sulfide concentrations increase. In many cases, a synergism exists between H2S and chloride. Separately, each will not cause SCC, but together they promote it.
The presence of elemental sulfur also increases susceptibility to SCC. The reason for this is complicated, but it is probably due in part to a shift in potential into a range in which cracking is observed. If the pH is decreased, the material becomes more susceptible to SCC. The tendency of a material to exhibit SCC is also very temperature dependent. As temperature increases, susceptibility to SCC in production environments also increases. Unfortunately, this is where corrosion-resistant alloys are of most use, because corrosion inhibitors often become ineffective at elevated temperatures.
Comparison of SSC and SCC
Stress-corrosion cracking is usually considered to be an anodic process, while SSC is considered to be a cathodic process. This can be very important in analyzing failures because the success of the method chosen to eliminate future failures may depend to a great extent on the determination of the proper failure mechanism. For example, it may have been decided that a particular failure was due to chloride SCC, and cathodic protection is being considered to increase the resistance of the material.
However, if the actual failure mechanism is SSC, this remedy may actually worsen the situation because SSC is a cathodic process. The application of a cathodic-protection current will tend to add even more hydrogen to the lattice, which will increase the likelihood of another failure. It should be remembered that failures of low-alloy steels will most often be the result of SSC, while the high-temperature embitterment of high-alloy stainless materials will most likely be due to SCC. Failure classification of intermediate alloys is sometimes more difficult.
Another important difference between SCC and SSC is the effect of temperature. Decreasing temperature usually causes a decrease in SCC susceptibility, while decreasing temperature causes an increase in SSC susceptibility. This can be useful when trying to determine the actual failure mechanism.


















Post a Comment